Terms like “green hydrogen”, “electrolysis”, and “hydrogen electrolyzer” are gaining prominence. As we described in our recent blog on hydrogen transport, electrolysis systems are popping up worldwide, and many governments are betting big on implementing green hydrogen as part of the transition towards renewable energy.
But how exactly is green hydrogen made? What is electrolysis, and what makes this technology sustainable?
In this blog, we answer these questions in detail.
Gray, blue and green hydrogen
While we have already extensively discussed the difference between green, blue and gray hydrogen in our blog on liquid hydrogen (LH2), we would like to briefly mention these three production processes again.
Gray hydrogen
Gray hydrogen is produced by steam methane reforming. The method uses hot steam to produce hydrogen from a methane source, such as coal or natural gas. Under the pressure of 3-25 bar, the two materials react when a catalyst is added, and this reaction then produces CO, CO2, and hydrogen.
To produce pure hydrogen, a “water-gas-shift-reaction” is created, from which heat, CO, and hydrogen are generated. The production of gray hydrogen is efficient but, unfortunately, quite harmful to the environment because of the large amount of CO2 that is released.
Blue hydrogen
A more sustainable alternative to gray hydrogen is blue hydrogen. The production of blue hydrogen works the same way as that of gray hydrogen, but with this method, the vast majority of the CO2 is captured, stored, or reused. This makes the process significantly more sustainable but also more expensive because the infrastructures for capturing the freed CO2 require materials and energy.
Green hydrogen
While gray and blue hydrogen are produced through steam methane reforming, green hydrogen is produced through electrolysis. This production process does not release CO2, by which green hydrogen is the designated sustainable fuel and energy carrier of the future.

What is an electrolyzer for hydrogen?
Electrolysis is simply a chemical reaction that uses electricity to break down compound substances into singular or other compound substances.
This method can be applied to a wide range of substances but is currently best known for splitting water to produce hydrogen. Electrolysis of water was discovered as early as 1807 by Humphry Davy, who presented his findings to the Royal Society of London in the same year.
In water electrolysis, electrical energy is used to split water into hydrogen and oxygen. The oxygen is either released in the process or captured and used; the hydrogen may or may not be converted to liquid hydrogen (LH2) by using a liquefier.
The electrolyzer is a reverse fuel cell. A fuel cell generates electricity by combining hydrogen with oxygen, while electrolysis uses electricity to produce hydrogen and oxygen.
How does this work? An electrolysis system consists of a water container containing an electrolyte and two electrodes: a cathode (negative pole) and an anode (positive pole). A chemical reaction occurs on both sides of the electrolyte by directing an electric current to both electrodes. Hydrogen is released on the cathode side and oxygen on the anode side.
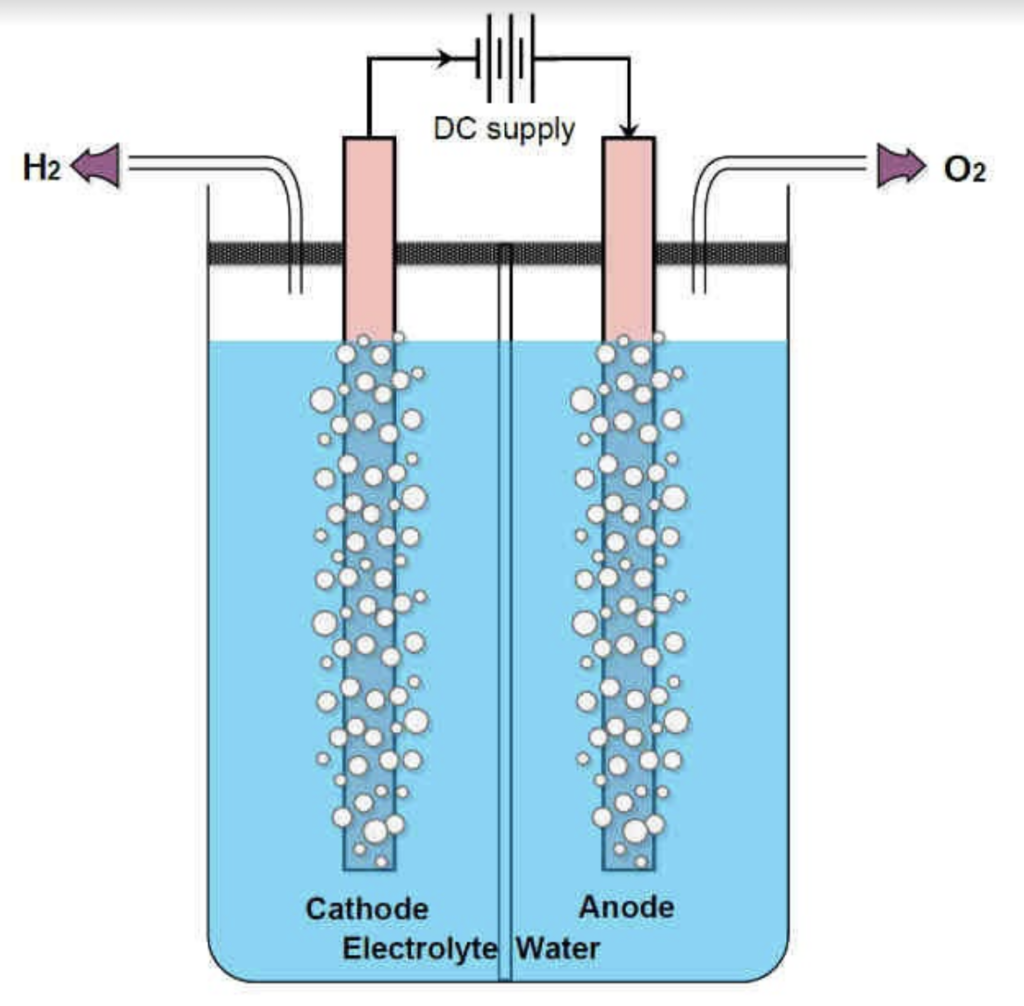
Source: International Journal of Engineering and Advanced Technology (IJEAT)
Obviously, the electrolysis process must use sustainably produced energy; this could include solar energy, wind energy, hydropower, or biomass. Electrolysis is still possible if this is not the case, but it can then not be considered green hydrogen.
Two types of an electrolyzer
The principle of electrolysis is well established, but there are different types of electrolyzers. The two best-known electrolyzers for hydrogen are the Alkaline electrolyzer and the PEM electrolyzer.
The alkaline electrolyzer
The alkaline electrolyzer uses a liquid alkaline electrolyte. The water in this electrolyte is split into hydrogen and hydroxide ions at the cathode pole. These ions are then brought into contact with a membrane, after which they are oxidized to water and oxygen at the anode pole.
A chemical reaction takes place at both the cathode and anode:
Cathode: 2 H2O + 2e → H2 + 2 OH-
Anode: 2 OH- → ½ O2 + 2e- + H2O
Total reaction: H2O → H2 + ½ O2.
About 285 kJ/mol of renewable energy is required to initiate this reaction (approximately 50 KJ/mol of heat and 235 kJ/mol of renewable electricity per mol of water). The temperature at which electrolysis occurs is between 40 and 90 °C.
Electrolyzers using liquid alkaline sodium or potassium hydroxide solution as the electrolyte have been available for a long time and have proven successful for hydrogen production. However, some disadvantages of this method include the relatively low current density of the cells and the fact that the cells cannot operate under high pressure and consequently are rather large.
The PEM electrolyzer
A PEM electrolyzer uses a polymer membrane that allows only hydrogen ions to pass through. The water is split into oxygen, hydrogen ions, and two electrons at the anode. The hydrogen ions and the two electrons then pass through the membrane and are converted to hydrogen at the cathode.
A chemical reaction takes place at both the cathode and anode:
Anode: H2O → H2 + ½ O2 + 2e
Cathode: 2H+ + 2e → H2
An advantage of the PEM electrolyzer over the alkaline electrolyzer is the fact that it also functions under pressure and, therefore, can be smaller in size. However, this method also has disadvantages. For example, the PEM electrolyzer operates under strongly acidic conditions, which demands special requirements for the system.
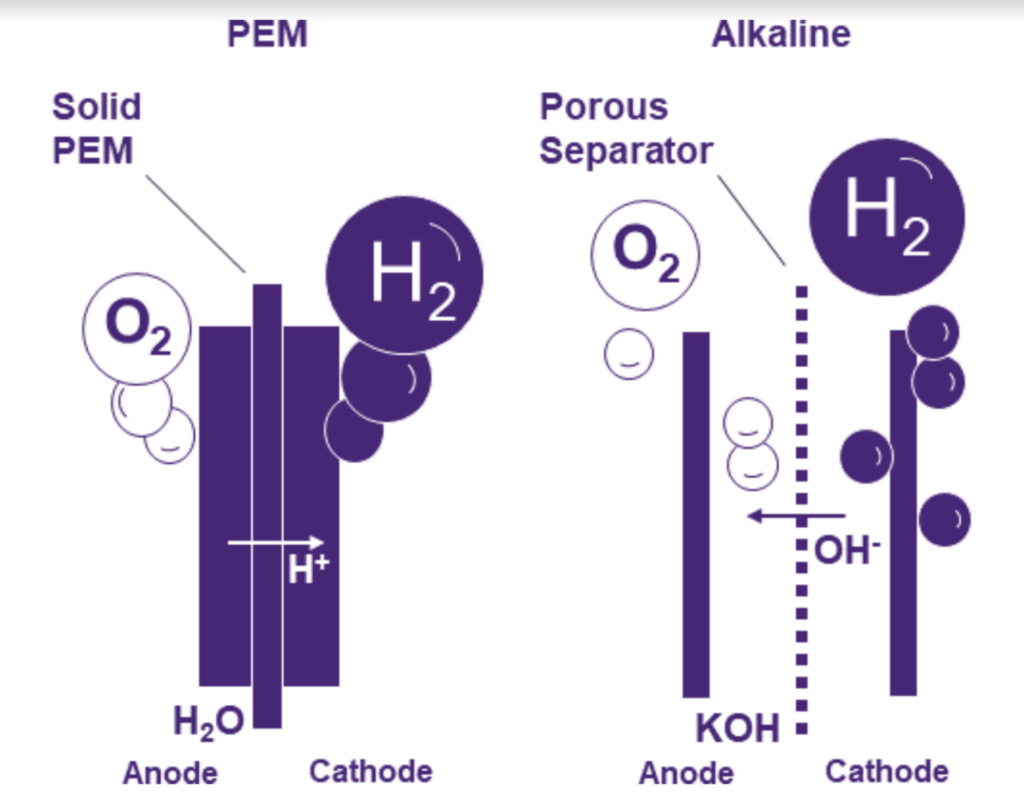
Source: Siemens
Do you want to know more?
Demaco has worked on the best liquid hydrogen (LH2) infrastructures for decades, and we design and produce vacuum insulated transfer lines and various auxiliary products. Demaco also carries out projects for the transportation, storage, and application of liquid hydrogen.
If you have any questions about our industry, please do not hesitate to contact us. or browse through our products and projects for more information.
Would you like to know more about liquid hydrogen (LH2)? Then take a look at this page or read our recent blog on “What is hydrogen used for?”. Here you can read all about this versatile cryogenic liquid and the many years of experience Demaco has gained within various state-of-the-art hydrogen projects.
